I. Introduction
Since the first purification of Nb, its nature has been the focus of scientists and engineers, and has been promoted in modern technology and industry. Indeed, with respect to other refractory metals concerned, Nb high melting point (2468 deg.] C), low density, corrosion resistance, and the superconducting properties of the oxide forming the dielectric capacity have led to its application in different fields.
Of course, the vast majority of applications are dependent on the purity of the niobium, which also depends on the purity of the potential utility, in particular the application of superconducting regard. In order to achieve higher purity, the scientific and technical personnel have made a lot of efforts, gained a lot of useful knowledge, and greatly improved the level of production know-how.
The emergence of electron beam smelting technology (EBM) has greatly promoted the production of base metals. The bismuth produced by this process has lower residual impurities than the conventional enthalpy obtained by solidification. In the late 1950s, HungR.Smith and Charles Hunt produced the first bismuth and antimony ingots on the Temescal equipment through EBM. Later, in 1959, Wah Chang began producing antimony and antimony ingots in an electron beam furnace.
The Nb-10%Hf-1% Ti alloy is an important high temperature bismuth based alloy that was first developed. Nb-10%Hf-1% Ti alloys have been used on high temperature components of turbine engines. Later, high purity Nb and some Nb alloys were applied in superconductivity. Nb-47% Ti alloy has always been the most demanding object of niobium-containing alloys, and it is used on superconducting magnetic poles, which are used in magnetic resonance imaging (MRI) units. At present, high-purity strontium powder has attracted extensive attention in the solid electrolyte industry as a potential competitor for hydrazine.
There are a number of technical documents currently involved in the production of tantalum, including the production process from refining metallurgy to casting the entire tantalum. Therefore, this paper aims to summarize the company's 12 years of experience in the production of commercial and reactor grade grade antimony ingots, from raw ore to final products.
 Second, issues related to purification
Pure tantalum has good toughness and elongation, and good low temperature processing properties, but only a few parts per million of oxygen, hydrogen or carbon impurities may make the metamorphosis hard and brittle, for some special purposes, such as Superconductivity, only a few ppm impurities are allowed.
In order to produce high-purity antimony ingots, electron beam melting technology is considered to be the most practical process at present, and its power density and protection of high or ultra-high vacuum are important factors for purification, and flexibility in controlling the residence time of molten metal. These factors are almost impossible to achieve for other vacuum metallurgical processes.
Under EB operating conditions, helium is purified by distillation and degassing. Elements that are higher than the vapor pressure are removed by evaporation. The Langmuir formula can be used to describe the specific evaporation rate. The formula shows the relationship between the evaporation rate and the partial pressure, molecular weight and temperature of each element. The formula is expressed as:
Where: a v1 (gcm - 2 s - 1 ) is the specific evaporation rate;
α is the evaporation coefficient - ideally α = 1;
P S (Pa) is the saturated vapor pressure at the T V temperature;
TV is absolute temperature;
MD is the molecular weight;
According to the Thumb principle, in order for purification to proceed effectively, the necessary conditions are that the vapor pressure of the impurity element should be at least 100 times the vapor pressure of the main element at the melting temperature.
Most of the impurities in the raw materials are mainly caused by the processing of Ara × a pyrochlore (Note: Ara × a pyrochlore is unclear), such as Al, Fe, Ti, Mn, Ba, Ka, Si and many others. Metals, their vapor pressures are much higher than the vapor pressure of helium. As can be seen from Figures 1 and 2, at the melting temperature of the crucible, these residual elements are relatively easy to evaporate from the molten crucible and are collected by the water wall and condenser in the melting chamber.
Figure 1 Vapor pressure curve of different elements
Figure 2 Evaporation rate of pure elements
For example, in the production of Nb-1% Zr alloy, it usually takes two steps of EB melting, and about 30% of the zirconium added to the crucible is lost by evaporation, while the recovery of rhodium is as high as 96%-98%.
On the other hand, low vapor pressure elements such as helium, tungsten and molybdenum cannot or hardly be removed from the crucible in the EBM process, so once present in the original ore, they have to be removed by other processes.
The low vapor pressure metal elements contained in CBMM's mines are only bismuth, and the strontium content level is consistent with the enthalpy contained in many commercial bismuth metals. The presence of tungsten and molybdenum is almost negligible. Therefore, when CBMM chooses raw materials to produce tantalum, in addition to carbon, oxygen and nitrogen, tantalum is the only metal element that requires special attention. It should be mentioned that the main components may also be evaporated. In the processing of niobium, the yield loss of niobium is about 1% to 3% due to evaporation of niobium. Therefore, it is normal for a low vapor pressure element such as cerium to have a small increase in concentration.
Figure 2 shows the approximate evaporation rate curves for some of the elements obtained under the ideal evaporation conditions using the Langmud formula, which is a qualitative description of the evaporation rates of other different elements.
Nitrogen, hydrogen and carbon monoxide are released from the molten material in the form of a gas and must be withdrawn by a vacuum pump. Therefore, in addition to achieving a very low final pressure, the furnace vacuum system must have sufficient pumping capacity to handle these gases. On the other hand, oxygen can be released from the molten ruthenium in the form of volatile low-valent metal oxides (mainly NbO and NbO 2 ) or combined with carbon (CO). For oxygen concentrations below 1%, deoxygenation with NbO is the primary method. Low-valent metal oxides volatilize from the molten pool and solidify in the water wall and condenser of the melting chamber.
Decarburization in bismuth mainly depends on excess oxygen in the sample. Therefore, if the carbon content in the first step of purification is not reduced to the desired level, it will be disadvantageous for further high vacuum remelting purification. In this case, another method is to re-melt under vacuum and with a higher partial pressure of oxygen. This process may increase the oxygen content.
Although this process is carried out under high vacuum, helium still has the ability to react with residual gases such as oxygen, nitrogen, carbon monoxide, carbon dioxide and water vapor. Depending on the partial pressure of these gases in the melting chamber, the reaction may be quite intense. Therefore, the final pressure in the melting chamber is a key factor for purification.
The thermodynamics, kinetics, and interstitial positional concentrations of hydrazine and gas reactions have been discussed in Ref.
Third, the EB furnace charge
The flexibility and electron beam controllability of modern EB furnaces allows the use of many forms of raw materials: sponge-like, compressed powder, block and rod.
For the production of niobium, the most important raw material is the rod type, which is obtained by reducing the niobium oxide of Al and C. Aluminothermic reduction technology is the most important technology used to produce EB furnace raw materials due to its high activity, low cost, and ease of preparation of carbon-free crucibles. The carbothermal reduction of ruthenium oxide is also an important method for the production of charge. In general, compared to the aluminothermic process, the raw materials produced by this method have lower residual oxygen and higher yield after EB smelting. However, carbon may react with ruthenium and osmium to form very stable compounds - Nb2C, NbC, TaC - which have melting points much higher than the melting point of pure ruthenium. Once these compounds are present in the feedstock, as mentioned earlier, in the EB process, decomposition and carbon removal will depend on the oxidizing power. Therefore, the process parameters of the carbothermal reduction process must be strictly controlled, especially the relevant stoichiometry, otherwise high-carbon content antimony ingots are obtained; on the contrary, impurities produced by the aluminothermic process are more easily removed during the EB melting process.
The charge required for the aluminothermic process is a mixture of cerium oxide and aluminum powder, and may also contain an activator and a flux. In order to ensure that 3% to 5% of aluminum can remain in the aluminothermic reduction crucible, the content should be tested. Practice has shown that excessive aluminum in the aluminothermic reduction of the bar makes the bar brittle and may rupture upon heating, which makes EB melting process control very difficult. Conversely, the lower the aluminum content, the more residual oxygen, resulting in a reduced yield. The oxygen content in the aluminothermic reduction crucible is normally in the range of 4000 to 8000 wppm.
In order to get the best results in the EB melting process, it is important to consider the chemical composition and particle size of the cerium oxide during the pre-selection process. Low vapor pressure impurities - W, Ta and Mo - It is important to note that these elements cannot (or are almost impossible) removed from the crucible by the EBM process.
The refractory lining of the casting mold in the aluminothermic process may also introduce unnecessary impurities. Special care should be taken when using carbon-containing refractories because it may increase the carbon content of the cast slab. On the other hand, CaO or MnO in the refractory material may contaminate the base metal, although they are not difficult to remove in the EBM process (due to its high vapor pressure), but may also reduce the yield.
Figure 3 shows the entire process of producing antimony ingots on Brazil CBMM equipment:
Figure 3 CBMM production flow chart
The first step in the melting of EB is the horizontal melting of the aluminum hot reduction crowbar. The melting rate must be adjusted according to the amount of gas in the raw material, the vacuum requirement in the melting chamber, the diameter of the furnace, and the effective power. For example, for a 500 kW furnace in CBMM, the furnace is equipped with a pump capable of pumping 50,000 liters per second, the melting rate is between 40 and 50 kg/hr, and the pressure in the melting chamber is 5 × 10 -4 - Change between 3 × 10 - 3 mbar. During horizontal feeding, the area of ​​the bath should be below the top of the feed rod and the electron beam cannot be reached unless the copper furnace is destroyed. Due to the continuous low heat flow, the quality of the corresponding areas of the antimony ingot will decrease, especially the residual of oxygen and aluminum and the smoothness of the surface. Figure 4 is a simplified diagram of the first step of EB melting.

Figure 4 CBMM company's first step of smelting operations
1-electron gun; 2-electrode; 3-vacuum chamber; 4-water-cooled mold; 5-retractable mold
In order to reduce the above negative effects, the solution is to cast a large cylindrical aluminothermic reduction crucible (200 mm in diameter, 1250 mm in length, each weighing about 300 kg) to accommodate vertical feeding. In the case of improving the quality of the ingot and increasing the high uniformity of the oxygen content in the transverse direction of the ingot (always below 300 wppm), the effect of the vertical instillation of the ingot produced is good. However, because of the tendency of the aluminothermic reduction crucible to thermally crack, heavy fragments may fall into the molten pool, resulting in a lower yield of crucibles and damage to the furnace, which is generally not used in normal production.
In general, in order to meet the ASTM-B391-96 reactor grade specifications, the EB smelting step must be repeated two to three times. Therefore, in the subsequent remelting, the ingot produced in the previous step is used as an electrode for vertical drip melting. Since the content of these electrodes is lower than that of the aluminum thermal reduction pry bar, the melting rate in the second and third steps can be higher.
Figure 5 is a vertical smelting equipment diagram
Production of bismuth ingots with a diameter of 250 mm (10 inches) on a 500 kW EB furnace. The process data is listed in Table 1.
Table 1 500kW electron beam furnace - operational data
Melting | raw material | size /mm | Melting speed /kg·h - 1 | Electron gun power / kW | Melting chamber pressure / m · bar | Average yield (w/w)/% |
1 st | Aluminothermic reduction crucible | 110×110 × 800 | 40-50 | 320-350 | <3 × 10-3 | 84 |
2 nd | a smelting rod | Φ250 dia.× 1,600 | 60-65 | 390-420 | <3 × 10-4 | 97 |
3 rd | Secondary smelting | Φ250 dia.× 1,600 | 65-70 | 420-440 | <5 × 10-5 | 98 |
Since 1989, CBMM has continuously produced pure niobium and Nb-1% Zr ingots, which meet the ASTMB391 standard shown in Table 2.
Table 2 ASTM B-391-96 Specifications for Nb and Nb Alloys
Ingredient requirements
element | Type I (reactor level is not combined Jinhua R04200 | Type II (commercial grade unalloyed 铌) R04210 | Type 3 (Reactor grade Nb – 1% Zr) R04251 | Type 4 (Commercial grade Nb – 1% Zr) R04251 |
Unless otherwise stated, generally refers to the maximum mass percentage |
Each ingot |
C | 0.01 | 0.01 | 0.01 | 0.01 |
N | 0.01 | 0.01 | 0.01 | 0.01 |
O | 0.015 | 0.025 | 0.015 | 0.025 |
H | 0.0015 | 0.0015 | 0.0015 | 0.0015 |
Zr | 0.02 | 0.02 | 0.8 to 1.2 | 0.8 to 1.2 |
Ta | 0.1 | 0.2 | 0.1 | 0.5 |
Fe | 0.005 | 0.005 | 0.005 | 0.005 |
Si | 0.005 | 0.005 | 0.005 | 0.005 |
W | 0.03 | 0.05 | 0.03 | 0.05 |
Ni | 0.005 | 0.005 | 0.005 | 0.005 |
Mo | 0.010 | 0.02 | 0.02 | 0.02 |
Hf | 0.02 | 0.02 | 0.02 | 0.02 |
When required |
B | 2 ppm | ----- | 2 ppm | ----- |
Al | 0.002 | 0.005 | 0.002 | 0.005 |
Be | 0.005 | ----- | 0.005 | ----- |
Cr | 0.002 | ----- | 0.002 | ----- |
Co | 0.002 | ----- | 0.002 | ----- |
The statistical data presented in Figures 6-11 are the compositional analysis reports and hardness results of the CBMM laboratory from 1994 to 2000. During this period, the company has produced more than 400 tons of antimony ingots for various purposes. This series of graphs gives a picture of the often changing elements. Most of the other unreported elements are below the lower limit that the analytical device can detect.
Figure 6 Carbon analysis of the first melted Nb ingot in 1998
Figure 7: First melted Nb ingot oxygen analysis in 1998
Figure 8 Nb ingot oxygen analysis after three times of smelting in 1994-2000
Figure 9 Nb ingot nitrogen analysis after three times of smelting in 1994-2000
Figure 10 Analysis of reactor-grade Nb ingots from 1994 to 2000
Figure 11 Nb ingot hardness after smelting three times in 1994-2000 (HV10)
Consumer and laboratory studies around the world confirm that most of the interstitial elements found in CBMM's antimony ingots are much lower than the ASTMB391 standard. Tables 3 and 4 list the GDMS (Glow Discharge Mass Spectrometry) analysis report. The samples used in Table 3 are from the same ingot after the first, second and third EB smelting; Table 4 shows the results of the component analysis after three different ingots after three EB smelting. It can be seen that, except for the unanalyzed gas and the aluminum in the first melted ingot, all other residual impurities have met or fallen below the ASTM B391-36 standard after the first EB smelting. CBMM produces enamels for all end users, including superconducting cavities, MRI and NMR poles.
Table 3 GDMS analysis results after 3 EBMs of the same bismuth ingot (ppm wt)
Table 4 Results of GDMS chemical composition analysis after 10 EBMs on 10563/01 and #10573/01 respectively (ppm wt)
   Fourth, summary
The highest purity is achieved by EB smelting and purification, and its purity has exceeded most of the current commercial requirements. Most of the metal elements in antimony ore are easily evaporated in an EB smelting environment. A very small amount of impurity elements having a saturated vapor pressure lower than that of ruthenium, such as ruthenium, tungsten, and molybdenum, cannot be removed by evaporation. Therefore, if the ore is selected correctly, most of the residual impurities in the EB process will be less than a few parts per million or even parts per billion.
Of the known methods for purifying base metals, none of them can exceed EB melting.
The rare-earth metal thick film heating technology is one of
today's most innovation and forward-looking solution for electric heating field.The
thick film heating tubes/elements are produced by screen-printing dielectrics
(5 layers), resistance (palladium-silver), conductor (silver) and isolation
glazes on the substrate and sintered 7-9 times at temperature over 900℃.
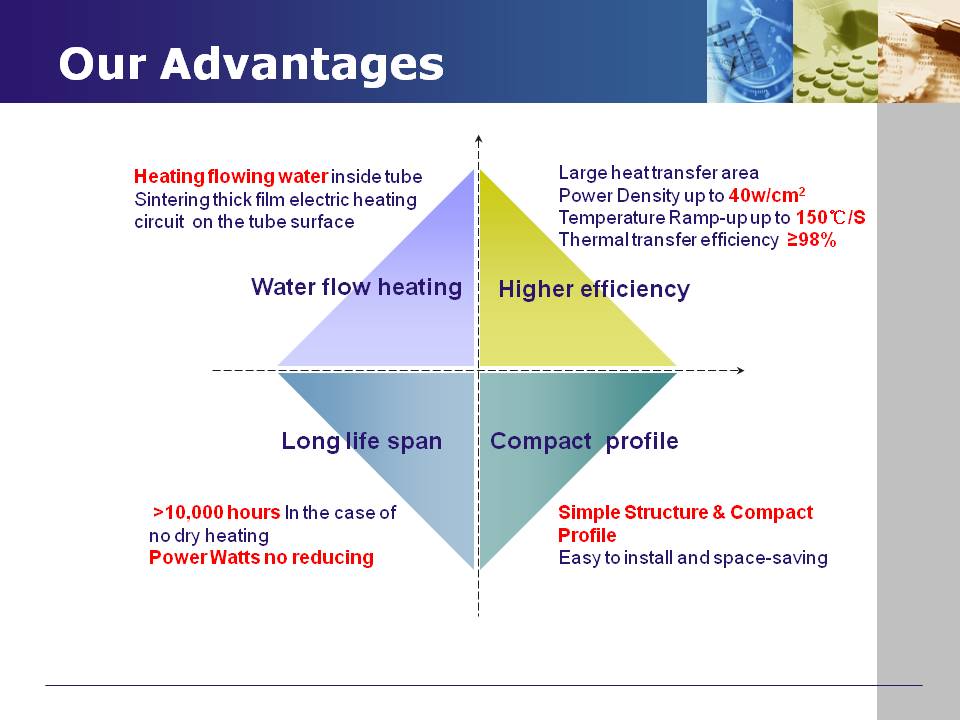
With 4KW Thick Film Heating Tube simple tubular structure,
it can heat water flow inside the tube. Electric Circuit printed on tube
surface, to heat the water flow inside. For applications where space is limited, this simple profile
heater offers high thermal power density and fast response times to heat up or
cool down (due to low thermal mass)

JIEDA company can supply products according to customers'
drawings and offer our clients reasonable prices. Our company also can develop
heating solutions and products together with customers.
Those new types Thick Film
Heater Tubes are special designed for Through-Flow applications, such as instant water heaters, instant water flow heater under
sink, conmmercial vendor and industrial water flow heater.

4KW Thick Film Heating Tubes
4000W Thick Film Heating Tubes,Infrared Shop Heater ,Infared Tube Heater ,Natural Gas Radiant Tube Heaters
XINXIANG JIEDA PRECISION ELECTRONICS CO.,LTD , https://www.tubularheater.de
















